Fast and Efficient Raman Microspectroscopy
- Abstract number
- 50
- Presentation Form
- Oral
- DOI
- 10.22443/rms.elmi2021.50
- Corresponding Email
- [email protected]
- Session
- Live and Functional Imaging Technologies Part 2
- Authors
- Alejandra Zegarra-Valverde (1), Walter Hauswald (1), Rainer Heintzmann (1, 2, 3)
- Affiliations
-
1. Leibniz Institute of Photonic Technology, Albert-Einstein-Straße 9, 07745 Jena, Germany
2. Institute of Physical Chemistry and Abbe Center of Photonics, Friedrich-Schiller-University, Jena, Germany
3. Faculty of Physics and Astronomy, Friedrich-Schiller-University, Jena, Germany
- Keywords
Raman imaging, light sheet microscopy, integral field spectroscopy, hyperspectral imaging
- Abstract text
In order to make Raman microspectroscopy faster and more efficient, a novel technique is presented. It combines light sheet illumination and integral field spectroscopic detection allowing the spectrally resolved simultaneous detection of 50×50 pixels. This system is capable to image polymeric samples with a detection time of 30 seconds and biological samples in 5 minutes. It is applied to identify contaminations of microplastics in TPH1 cells.
Raman microspectroscopy allows the characterization of living samples providing intrinsic molecular specificity without labeling or staining. However, due to its low efficiency and absorption-based sample heating [4], traditional Raman imaging approaches (e.g. confocal microscopy) fail to acquire fast high-quality images and lead to vast phototoxicity.
Previous techniques based on light sheet illumination avoided unnecessary out of focus illumination, which reduced sample heating. In combination with a Fourier-transform imaging spectrometer, the full hyperspectral image acquisition was shown to increase by a factor of five compared to confocal approaches [1]. The acquisition was limited by the signal-to-noise ratio of the detection system.
Here we present a new approach combining light sheet illumination with hyperspectral imaging, meeting both optimization criteria: low light load and high signal to noise ratio. The integral field-based [2, 3] spectroscopic detection records 50×50 spectra in parallel. Thus, an enhancement in acquisition speed by a factor of 2500 compared to confocal methods can theoretically be achieved [4], which enables qualitatively new applications in biomedical as well as clinical research.
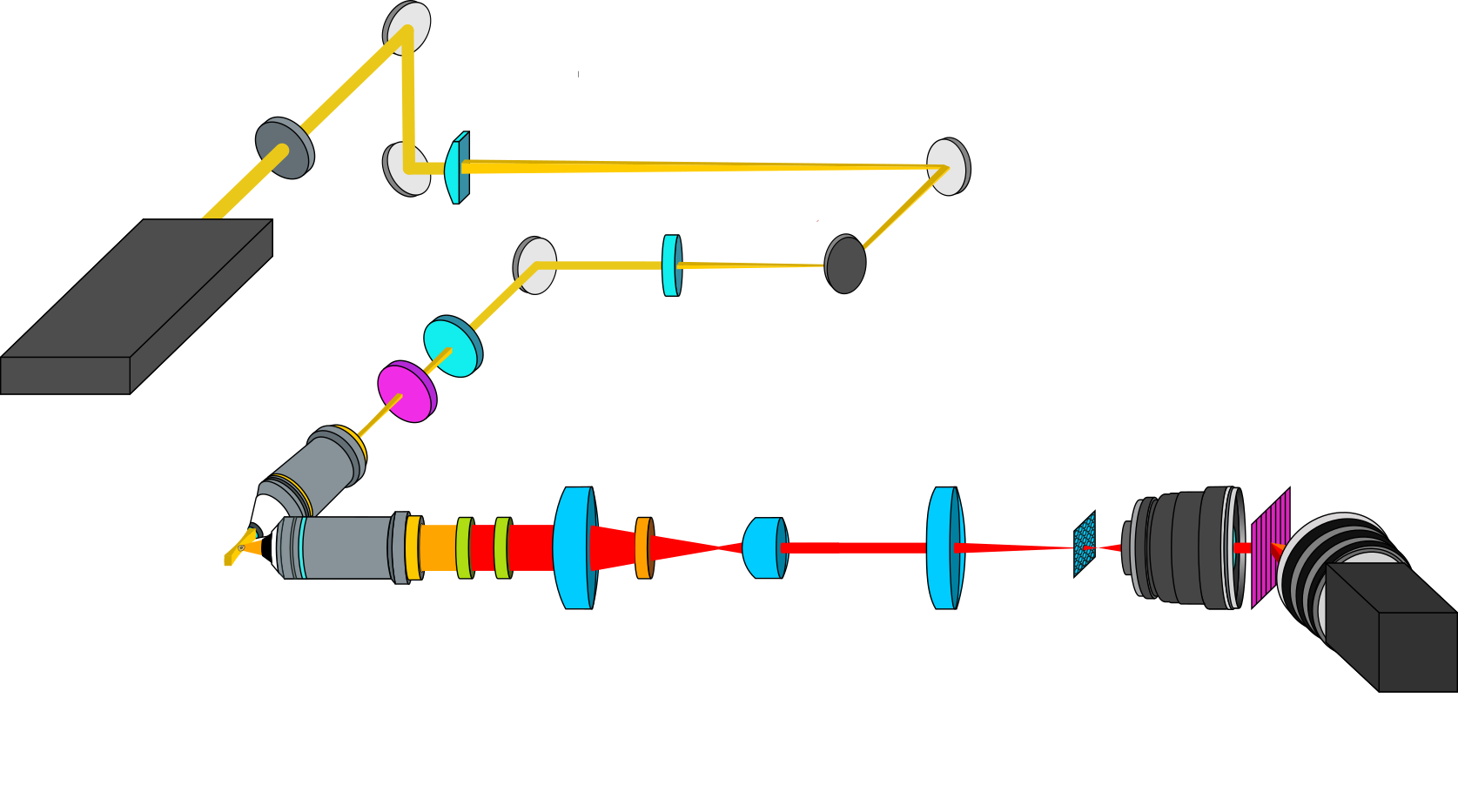
The experimental setup is shown in Figure 1. The illumination path consists of a modified OpenSPIM [5] system using an excitation wavelength of 577 nm. A 4 µm thick and 50 µm high light sheet created by a series of cylindrical lenses sections a single plane of the sample. The latter is fixed with agarose and located in a water chamber, suitable for biological applications. For the detection, the object is imaged and Rayleigh filtered by a two-stage system with an overall magnification amounting to 128 onto a microlens array for resampling. The resampled image is finally re-imaged by a spectrograph consisting of two photographic objectives and a grating onto a CCD camera. The acquired spectral resolution amounts to 4 cm-1 over a range from 700 cm-1 to 1700 cm-1 and the spatial resolution is 400 nm.
Figure 1
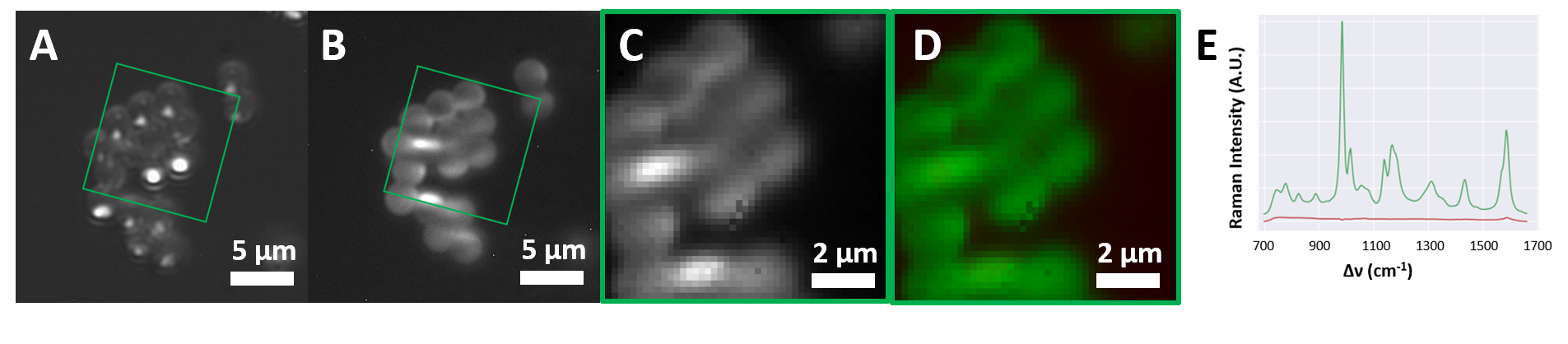
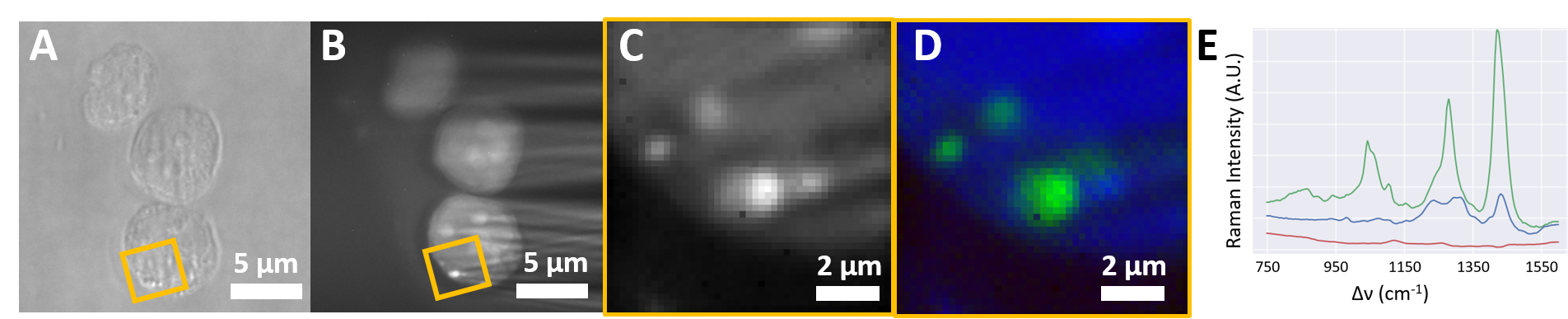
Raman hyperspectral images of polystyrene microbeads (30 s acquisition time) and TPH1 cells fed with microplastics (5 min acquisition time) are presented in Figure 2 and Figure 3 respectively. (A) and (B) show the bright field and the spectrally integrated light sheet Raman images, respectively. (D) shows the images (highlighted in (A) and (B)) after non-supervised spectral unmixing. Different detected components are color-coded: Green areas in Figure 2 are attributed to polystyrenes spectra of the beads. Green and blue areas in Figure 3 are assigned to microplastic in and cytoplasm of the TPH1, respectively. The corresponding measured spectra are depicted in (E).
Figure 2
Figure 3
It was shown that this technique is able to acquire a Raman image of cells with an exposure time of minutes. This method will be applied to characterize single cells for the investigation of intracellular bacterial pathogens such as chlamydia.
- References
[1] W. Müller, M. Kielhorn, M. Schmitt, J. Popp, and R. Heintzmann, "Light sheet Raman micro spectroscopy", Optica, 3, 452–457 (2016).
[2] R. Bacon et al., "The SAURON project-I. The panoramic integral field spectrograph", Mon. Not. R. Astron. Soc. 326, 23-35 (2001).
[3] A. Zegarra-Valverde, W. Müller, and R. Heintzmann, "Integral Field Light Sheet Raman Microspectroscopy,", Frontiers in Optics + Laser Science APS/DLS, OSA Technical Digest (Optical Society of America, 2019), paper FM3F.5. (2019).
[4] W. Hauswald, R. Förster, J. Popp, R. Heintzmann, "Thermal illumination limits in 3D Raman microscopy: A comparison of different sample illumination strategies to obtain maximum imaging speed", PLOS ONE 14(8) (2019).
[5] P.G. Pitrone et al., “OpenSPIM: an open access light sheet microscopy platform”, Nat. Methods in press (2013).