Application of low-cost stochastic optical reconstruction microscopy to the histological analysis of human glomerular disease
- Abstract number
- 28
- Presentation Form
- Poster Flash Talk and Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 2
- Authors
- Edwin Garcia (1), Jonathan Lightly (1), Sunil Kumar (1, 2), Ranjan Kalita (1), Frederik Görlitz (1), Yuriy Alexandrov (1, 2), Terence Cook (1), Christopher Dunsby (1, 2), Mark Neil (1, 2), Candice Roufosse (1), Paul French (1, 2)
- Affiliations
-
1. Imperial College London
2. Francis Crick Institute
- Keywords
super resolution; immunofluorescence microscopy; STORM, histology, pathology
- Abstract text
Summary
Electron microscopy (EM) is used for diagnosis in human glomerular diseases in the UK and elsewhere but diagnostic EM is not available in many countries. Single molecule localisation microscopy (SMLM) can extend conventional immunofluorescence of stained tissue to resolution near EM. We are exploring the diagnostic value of easySTORM, our low-cost implementation of dSTORM, applied to standard histological sections of frozen and paraffin-embedded clinical kidney samples.
Introduction
Electron microscopy (EM) following immunofluorescence (IF) imaging is established in the UK for the diagnosis of human glomerular diseases but the implementation of EM is limited to specialised institutions and it is not available in many countries. We have applied easySTORM [1] , our low-cost implementation of dSTORM [2] to upgrade a standard widefield fluorescence microscope to provide immunofluorescence images with resolution below 50 nm starting from standard histological sections from human kidney biopsies - both frozen and formalin-fixed and paraffin-embedded (FFPE) – to explore whether this may provide an alternative to EM for diagnosing kidney disease. We have developed a workflow that we designate “histoSTORM”, utilising clinically approved immunofluorescent probes for the basal laminae and immunoglobulin G deposits and have compared this approach to clinical electron microscopy images. We demonstrate enhanced imaging compared to conventional immunofluorescence microscopy for cases of membranous glomerulonephritis, thin basement membrane lesion and lupus nephritis. Thus minor modifications of established immunofluorescence protocols for clinical renal biopsies may enable a cost-effective alternative to EM to aid diagnosis of human glomerular disease.
Methods
“histoSTORM”: immunofluorescence staining was carried out on frozen and formalin-fixed paraffin embedded (FFPE) tissue from renal biopsies with Membranous glomerulonephritis (MGN), Lupus nephritis and Minimal change disease. Staining was performed for IgG documented with ifluor 647 and for Glomerular Basement Membrane (GBM) laminin documented with Alexa Fluor 555 using antibodies in routine clinical use. dSTORM of these tissue sections was undertaken using our open source easySTORM adaptation of a conventional fluorescence microscope.
“histoSTORM” was applied to immunofluorescence of both FFPE and frozen histological sections using standard clinically approved antibodies. The super-resolved immunofluorescence images rendered at 25 nm per pixel reveal well-defined subepithelial deposits in MGN and enlargement of the GBM that are consistent with those observed by EM. In a case of stage IV lupus nephritis, mesangial, subendothelial and subepithelial IgG deposits are readily observed with histoSTORM and recapitulate the distribution of electron dense IgG deposits documented with EM. histoSTORM also enables GBM thickness measurements on paraffin-embedded tissue with resolution below diffraction in a case of Minimal change disease.
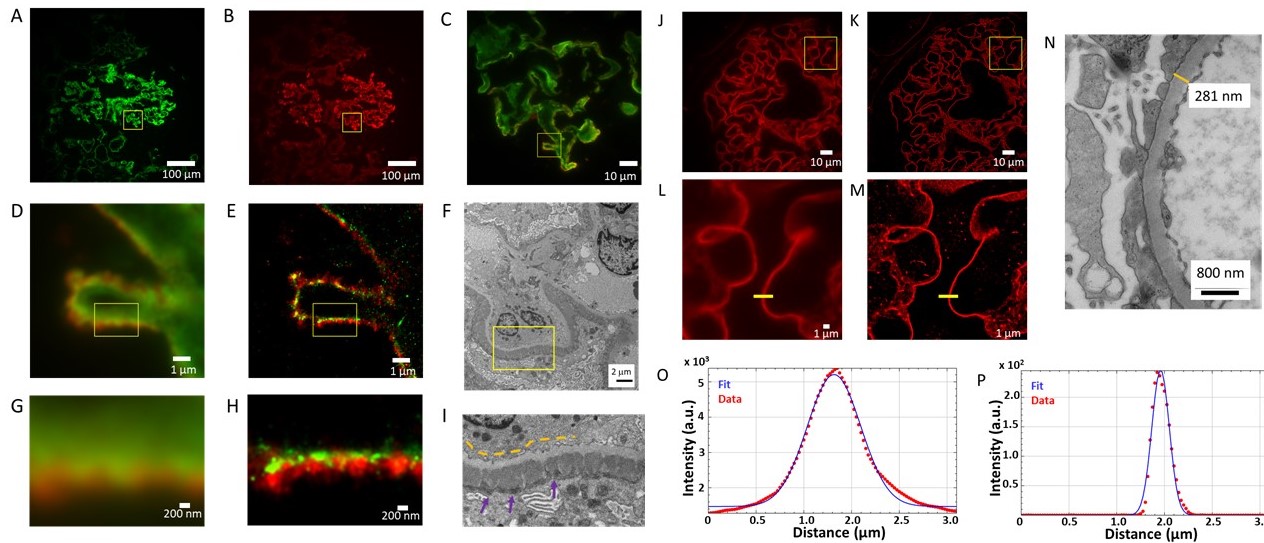
A-I: Basement membrane (laminin, green – Alexa Fluor 555), Immunoglobulin G deposits (IgG, red – iFluor 647). A-B. Widefield immunofluorescence images at 20x magnification of frozen section of Membranous Glomerulonephritis showing A: laminin channel,
B: IgG channel, and C: expanded two-channel image at 100X magnification of region indicated by yellow square in A and B;
D: Widefield immunofluorescence of region indicated by yellow square in C; and
E: corresponding STORM image with pixel size rendered at 25 nm.
F: Electron micrograph of similar structure from same biopsy at 20,200x magnification.
G: Widefield immunofluorescence image of 3.2 x 2.4 µm2 region indicated in D,E with H: corresponding STORM image;
I: expanded electron micrograph image of region indicated in F. Yellow dashed lines indicate the light grey glomerular basement membrane. Dark grey electron-dense deposits on the sub-epithelial side (purple arrows) represent immune complexes containing IgG.J-P: Glomerular Basement Membrane (Laminin-iFluor 647)
J: Widefield immunofluorescence image at 100x magnification of FFPE section
K: Rendered STORM image of region shown in J. L: Widefield inset of a region shown in J. M: STORM inset of region shown in K rendered with a pixel size of 25 nm.
N: Electron micrograph of a GBM from different section of same biopsy 60,700x magnification, for which the GBM thickness at indicated position is 281 nm.
O: Presenting measured thickness (full width at half maximum) of GBM from wide-field immunofluorescence image C line profile (657 nm)
P: Measured thickness (FWHM) of STORM image M at line profile (212 nm).Figure adapted from reference [3].
Conclusions
While this study [3] does not establish that histoSTORM can fully replace EM in renal diagnosis, it does provide evidence of added value relative to Light Microscopy and IF. The large (dSTORM) field of view (120 µm square) and standard sample preparation make it more convenient than EM (as well as more affordable). However, prospective studies of large case series are required to establish its clinical utility. histoSTORM may not be able to replace EM for all renal diagnoses but it may have potential for wide clinical impact, especially in less well-resourced settings where EM is not available. We note that STORM has previously been applied to research pathology, e.g. to study epigenetic modulation [4] and the progression of cancer [5] but not to clinical histological sections using clinically approved antibodies, to the best of our knowledge.
- References
[1] Kwakwa, K., et al.,. “easySTORM: a robust, lower-cost approach to localisation and TIRF microscopy,” J. Biophotonics, 9, 948–957 (2016).
[2] Heilemann, M., et al., Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angewandte Chemie (International Ed. in English), 47, 6172–6176 (2008)
[3] E. Garcia et al., “Application of direct Stochastic optical reconstruction microscopy to the histological analysis of human glomerular disease”, J Pathology: Clinical Research, in press
[4] Xu J, Ma H, et al., Super-Resolution Imaging of Higher-Order Chromatin Structures at Different Epigenomic States in Single Mammalian Cells. Cell Reports 24 (2018) 873-882.
[5] Xu J, et al., Super-resolution imaging reveals the evolution of higher-order chromatin folding in early carcinogenesis. Nature Communications 11 (2020) 1899.