A versatile and highly customisable platform optimised for live high/super-resolution imaging of invading multicellular tumour spheroids in 3D collagen matrices
- Abstract number
- 27
- Presentation Form
- Oral
- DOI
- 10.22443/rms.elmi2021.27
- Corresponding Email
- [email protected]
- Session
- Multiparameter and Multimodal Imaging for High Dimensional Data Acquisition
- Authors
- Tom Phillips (1), Maddy Parsons (1), Susan Cox (1)
- Affiliations
-
1. King's College London
- Keywords
live cell imaging, 3D, invasion, timelapse, tumour, spheroid, collagen, matrix, ECM, mechanobiology, mechanosensory, YAP, SoRa, super resolution, high resolution, PDMS, microfabrication, photolithography
- Abstract text
Summary
Imaging of multicellular tumour spheroids is a powerful approach to understanding tumour cell-ECM dynamics in an in vivo-like system. Inconsistent sample preparation and variable sample depth often hinder the scope of this approach due to objective lens working distances and sample density. We have developed a versatile method optimised for high resolution imaging of tumour spheroids which utilises microfabricated PDMS stamping of collagen-I hydrogels to allow precise positioning of spheroids in 3D ECMs. The stamping method was used to examine the effect of extracellular matrix stiffness on spheroid invasion and activation of the mechanoresponsive transcription factor YAP. Data revealed that YAP overexpression induced invasion and YAP inhibition/knockdown inhibited invasion of breast cancer cell spheroids. This method is versatile, optimised for imaging and is compatible with many standard stage inserts and imaging systems.
Introduction
Study of cancer cell invasion using multicellular tumour spheroids embedded into reconstituted extracellular matrices (ECM) represents a powerful approach to study tumours in an in vivo-like manner with great versatility achieved by using well-characterised cell lines or primary tumours as well as a variety of tuneable ECM types. However, understanding the finer scale dynamics of cell invasion, spheroid organisation, ECM remodelling and cell-ECM interaction often requires a higher resolving power which is mainly limited by objective working distance, sample depth and sample density.
Methods & materials
To circumnavigate inconsistencies with imaging of live tumour spheroid invasive dynamics in 3D, we have developed an experimental system on standard imaging chamber slides which utilises customisable, micropatterned PDMS stamps to generate patterned structures of consistent diameter/depth into collagen type-I ECMs with tuneable X-, Y- and Z-positioning for reproducible sample preparation and imaging of large, dense samples with high-/super-resolution systems. These structures can be stamped in any standard slide.
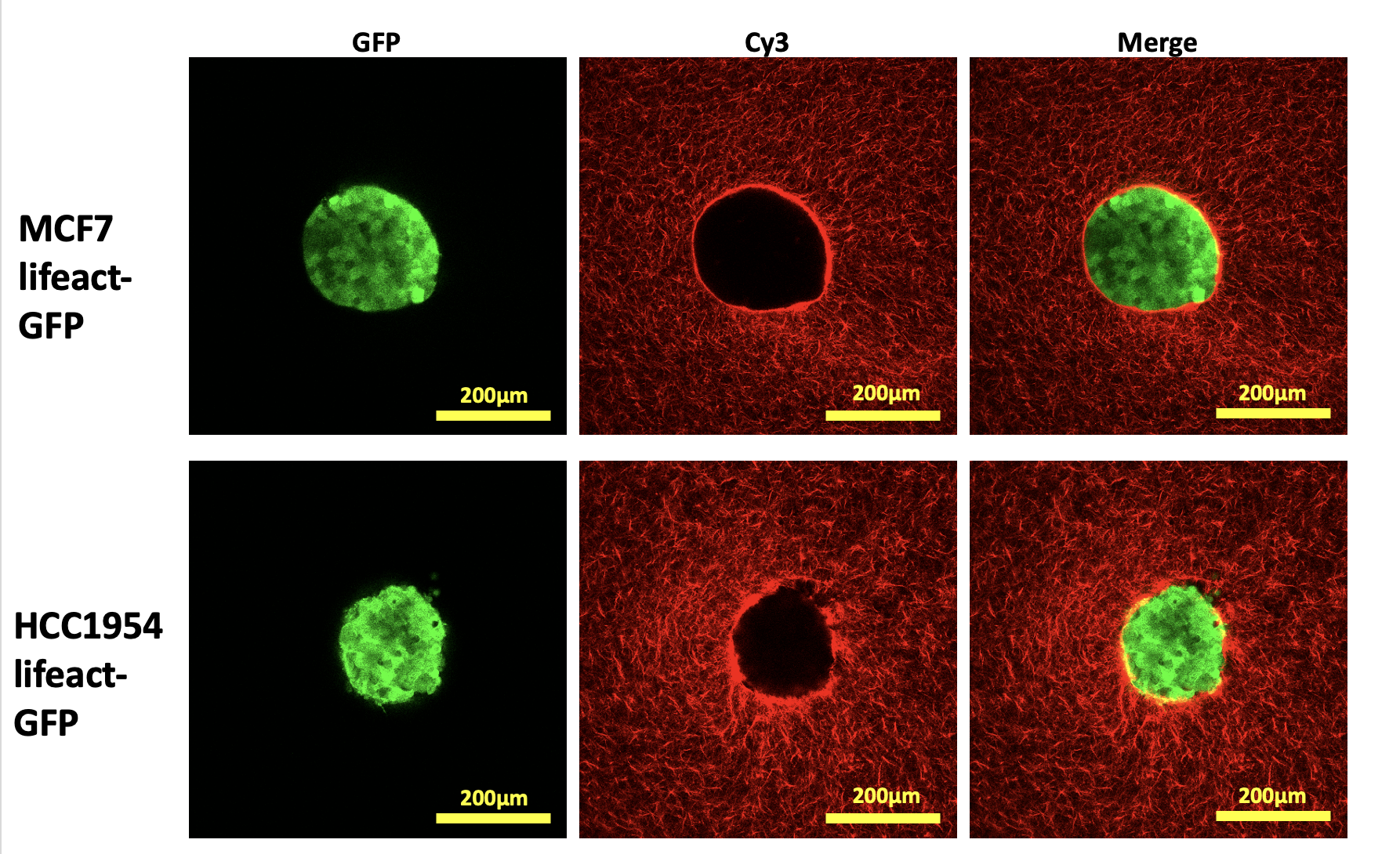
Supporting figure 1 – images of MCF7 and HCC1954 spheroids embedded in cy3-stained collagen within 200µm stamped collagen wells 1h post-seeding.
Spheroids of 400 cells for MCF7(non-invasive breast cancer cell line)and 750 cells for HCC1954 (invasive breast cancer cell line) were generated using the hanging drop method and embedded into stamped collagen gels pre-stained with Cy3 mono-reactive dye to examine cancer cell invasion into 3D collagen matrices over 5 days (Figure 1). Collagen gels were incubated with ribose solutions of varying concentration to alter matrix stiffness in concert with various collagen staining methods to allow for imaging of stiffness-dependent cell-ECM interactions, particle image velocimetry analysis and quantification of collagen dynamics with ‘The Workflow of Matrix BioLogy Informatics’ (TWOMBLI). Samples were imaged with point scanning confocal, W1 spinning disk and SoRa systems.
Results/discussion
The model system is relatively simple to set-up in a variety of different imaging chambers suited for inverted microscopy and has been utilised to study YAP-induced breast cancer cell invasion into 3D collagen matrices of varying stiffnesses. MCF7 spheroids overexpressing GFP-YAP invaded into stamped collagen matrices in a stiffness-dependent manner, while parental MCF7 cells did not invade. HCC1954 spheroids, expressing high levels of endogenous YAP, showed reduced invasion upon treatment with verteporfin, a YAP inhibitor, or following siRNA-induced YAP knockdown. MCF7 GFP-YAP and HCC1954 spheroids invaded predominantly collectively in a stiffness-dependent manner, with HCC1954 spheroids significantly degrading and remodelling ECM. High resolution imaging indicated that YAP activity was significantly higher in outer cells and that this was stiffness-dependent. PIV analysis of Cy3-stained collagen indicated that cells actively remodelled the matrix both locally and on larger scales. TWOMBLI analysis further demonstrated significant alignment of collagen fibres proximal to HCC1954 spheroids as well as at distances displaced outside the spheroid boundary.
Conclusion
We have developed a highly versatile platform for imaging live multicellular tumour spheroids, cell invasion and cell-ECM dynamics at high/super-resolution. Versatility arises from differing well dimensions, variable positioning in 3D space and altering ECM stiffness. The system has been used to show the mechano-sensing role of YAP in 3D cancer cell invasion in a variable stiffness environment, and the interplay between cells and ECM remodelling and alignment. We envisage this will provide an adaptable method for analysis of a broad range of tumour cell types, multi-cellular models and potentially for high resolution drug screening approaches in future.